
Assessment of Alcohol-Induced Dose Dumping with a Hydrocodone Bitartrate Extended-Release Tablet Formulated with CIMA® Abuse Deterrence Technology – topic of research paper in Clinical medicine. Download scholarly article PDF and read for
Predicting Responders to Reslizumab after 16 Weeks of Treatment Using an Algorithm Derived from Clinical Studies of Patients wit
Clinical and Economic Outcomes in Patients with Persistent Asthma Who Attain Healthcare Effectiveness and Data Information Set M
NDA 208082/S-002 SUPPLEMENT APPROVAL Teva Branded Pharmaceutical Products R&D, Inc. Attention: David Truong, PharmD, MS Asso
Investigating the Accuracy of the Digihaler, a New Electronic Multidose Dry-Powder Inhaler, in Measuring Inhalation Parameters

Biomedicines | Free Full-Text | Trends and Perspectives of Biological Drug Approvals by the FDA: A Review from 2015 to 2021
NDA 208798 NDA 208799 WRITTEN REQUEST – AMENDMENT 2 Teva Pharmaceutical Industries Ltd. c/o Teva Branded Pharmaceutical Produc
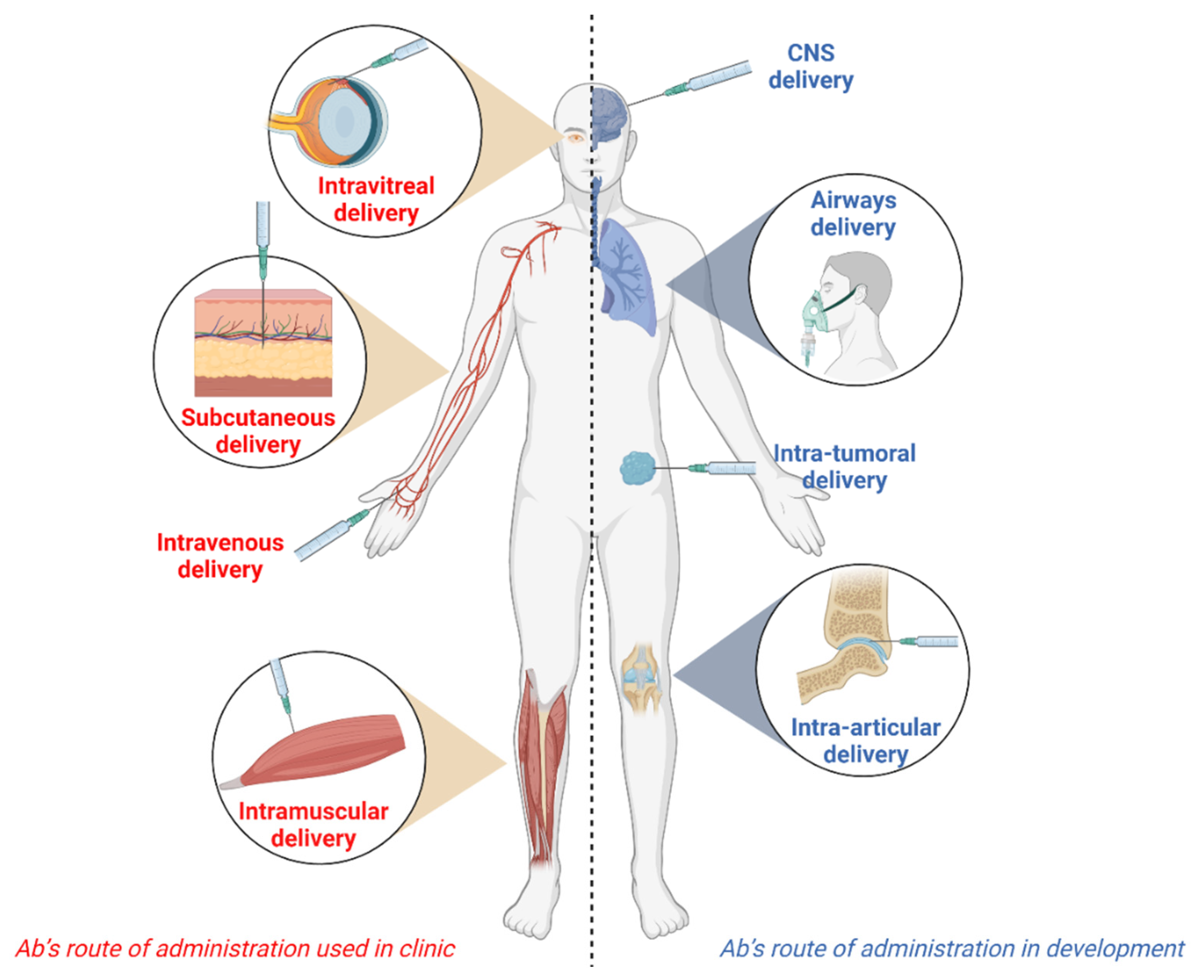
Antibodies | Free Full-Text | Alternative Routes of Administration for Therapeutic Antibodies—State of the Art

Teva Branded Pharmaceutical Products R&D;, . An electronic inhaler add-on module which store and transmit information related to inhaler use (eModule) PDR0000401 FCC ID 2AJVSPDR0000401

PDF) An evaluation of the potential for drug–drug interactions between bendamustine and rituximab in indolent non-Hodgkin lymphoma and mantle cell lymphoma






